 An introduction to
An introduction to
Life Sciences Packaging
Many of the breakthroughs that we often witness in the life sciences sector have been precursors to the changing face of cold chain logistics. Vaccines and insulin products, the rise of biologic-based therapies, as well as the demand for more speciality pharmaceuticals have all encouraged biological temperature control packaging development; combating disease at the cellular level continues to move us forward.
Guaranteeing the integrity in transit of temperature-sensitive products composed of sugars, proteins, nucleic acids, or live entities such as cells and tissues is a zero-tolerance business. Tempack has a range of isothermal biological packaging that has been conceived, designed, manufactured and rigorously tested for precisely this purpose.
Depending on your specific requirements, full kits or separate components can be utilised that will honour regulatory compliance for Biological Substances, Categories A and B. In packaging terms, this equates to UN2814 and UN2900 regulations for infectious or potentially infectious samples (embedded in P620 packaging guidelines) and UN3373 regulations for diagnostic substances (in the P650 guidelines).
Effective and protective, Tempack’s packaging components combine to safeguard shipments from the moment they leave the manufacturing site or laboratory to their final medical outpost. The key objective: the well-being of the patient.
Tempack isothermal biological packaging solution also meticulously adheres to the different prevailing international transport regulations: IATA (air transport), IMDG (maritime shipping), RID (rail transport) and ADR (road transport).

DSX Diagnosach
Extensive biological preservation
Reusable and with a good life expectancy, the Diagnosach DSX medical transportation kit is a lightweight, isothermal shipping container that has been specifically designed to overcome the difficulties of shipping biological samples under controlled temperature conditions. It also lends itself to the shipment of pharmaceuticals and chemical goods. The Diagnosach DSX medical cooler combines a quilted, waterproof overpack with a substantial, isothermal, internal container to protect goods at chilled, ambient and frozen levels for up to 4 days.

Patient Bags
Door-to-door stability
Pharmaceutical products, insulin, blood and vaccines will all make it home safely using Tempack temperature-controlled patient bags and covers. Lightweight and highly-portable, they will perform in different temperature ranges and a unique, triple-layer, high-density composition guarantees transportation autonomy for up to 12 hours. This depth of functionality has made these insulated bags useful for all kinds of temperature-sensitive goods. The materials used in Tempack cooler bags and covers mean they are easy to clean and sterilise.

ATP 650 Insulated Box
Collapsible thermal protection for Category B substances
The ATP 650 is high-performance, exterior, temperature control packaging for biological and potentially hazardous substances. UN 3373-certified, this strong, rigid, insulated, water-resistant system will regulate its temperature-sensitive contents, keeping them at either refrigerated or ambient temperatures, according to requirements. The Tempack ATP Insulated Box is an isothermal packaging solution for biological samples with a collapsible function that reduces storage and transport costs. It is designed to work with corresponding secondary packaging.
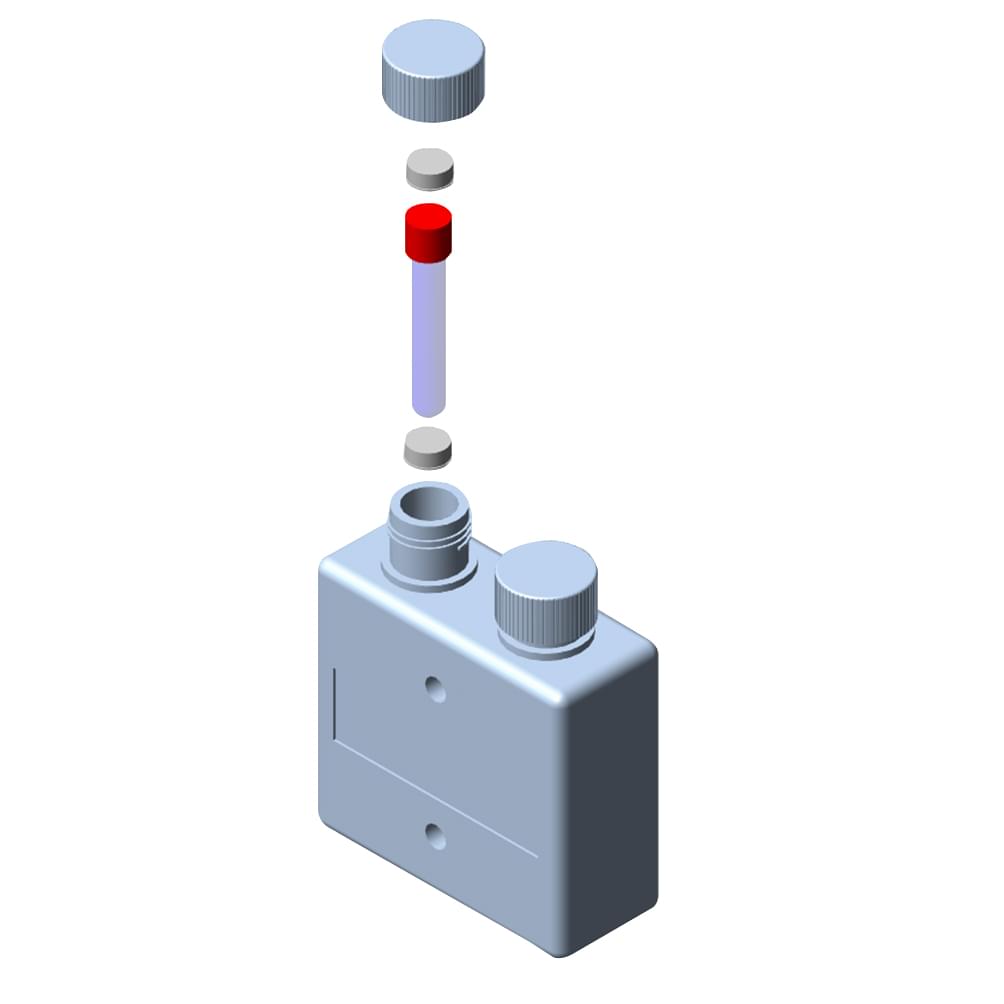
P650 Termovial
Reliable, reusable transport for vials at controlled temperatures
The P650 Termovial isothermal bio-container for test tubes and vials will protect the integrity of diagnostic samples for the full duration of their journey. Exterior packaging works with these sturdy, reusable vessels to safeguard products in transit at refrigerated or frozen levels, for up to 96 hours. Each diagnostic sample container is hermetically sealed, non-toxic and conforms with layers of UN and transport regulatory policy.
